PPE News Flash - Volume 5
Intertek PPE News
US CDC Updates Guidance on Masks and Respirators
The Centers of Disease Control and Prevention (CDC) has updated its guidance on masks and respirators to include more information on N95s and other medical-grade masks for the general public. An issue with commercially available high-filtration masks is that they may not come from reputable suppliers. CDC warns that about 60 percent of KN95 respirators available in the U.S. are counterfeit.
More Intertek’s PPE Centres of Excellence accredited to ASTM F3502
In addition to its US PPE Centre of Excellence, Intertek’s Shanghai and Guangzhou PPE Centres of Excellence have now been accredited to test against ASTM F3502 standards for barrier face coverings in the US market. Intertek customers who have fulfilled all mandatory tests or recommended requirements of international or national standards or guidance can apply for our Intertek Mask Label to showcase verified quality and performance attributes of mask products on our PPE CoE Directory.
PPE Recall Cases & Safety Alerts
EU Safety Gate
EU’s Safety Gate rapid alert system issued a total of 113 notifications related to filtering mask (112 notifications) and disposable gloves (1 notification) from Jan 1, 2021 till early October. Common failure issues of these two categories of Personal Protective Equipment (PPE) include PPE were not certified by an appropriate notified body and the products did not perform as claimed which was mainly due to poor filtration performance or the mask did not fit properly to the face.
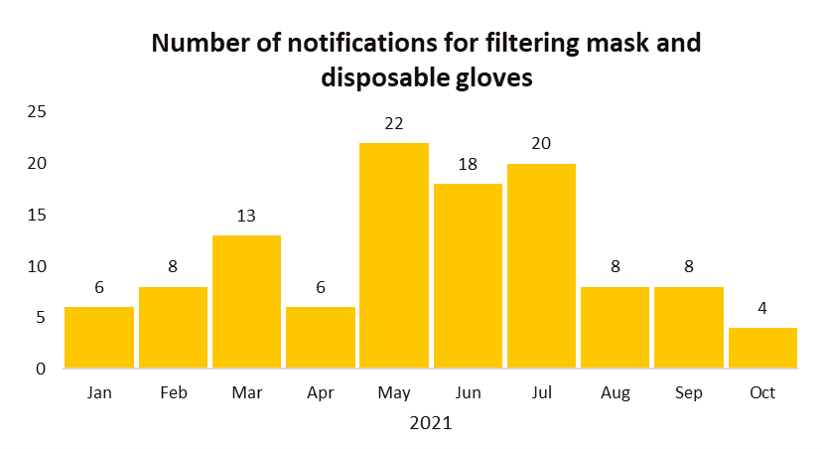
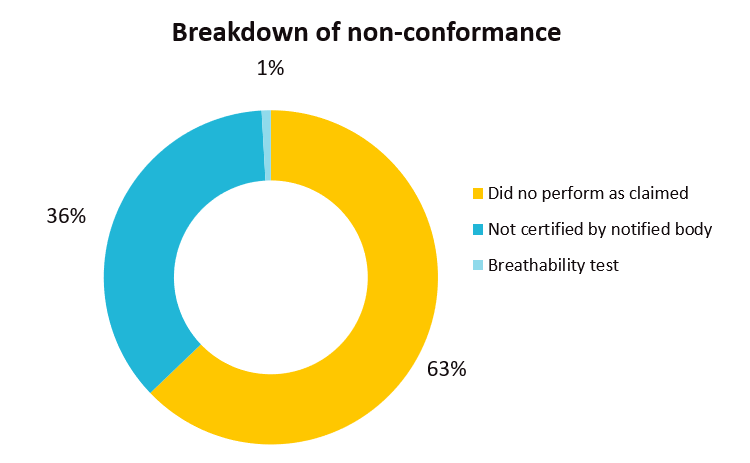
The recall cases demonstrate the importance of testing face masks to the appropriate standard(s) and ensuring the products meet the relevant performance requirements.
Regulatory Updates
Update of Spanish Specification for Hygienic Masks (Face covering)
The Spanish standardization body has updated their specifications for face coverings. New versions of specifications for hygienic masks have been released in September 2021. The updated versions below aim to provide a better alignment with the CSM/115/2021 guidance with regards to marking and instructions for use:
- UNE 0064-1:2021 Non-reusable hygienic masks Part 1: For Adults use
- UNE 0064-2:2021 Non-reusable hygienic masks Part 2: For Children use
- UNE 0065:2021 Reusable hygienic masks for Adult and Children
Hygienic masks must comply with the following basic principles:
- Should cover the nose, mouth, and chin
- Must be made of suitable filter material
- Must be made of breathable material
- Material should not present health risk
- Proper fit to the face
There are no major changes to performance requirements such as filtration or breathability (see table). The key changes allow manufacturers to create changes to design, shape or sizes of masks, provided they still need to comply with all a criterion stated within the specifications.
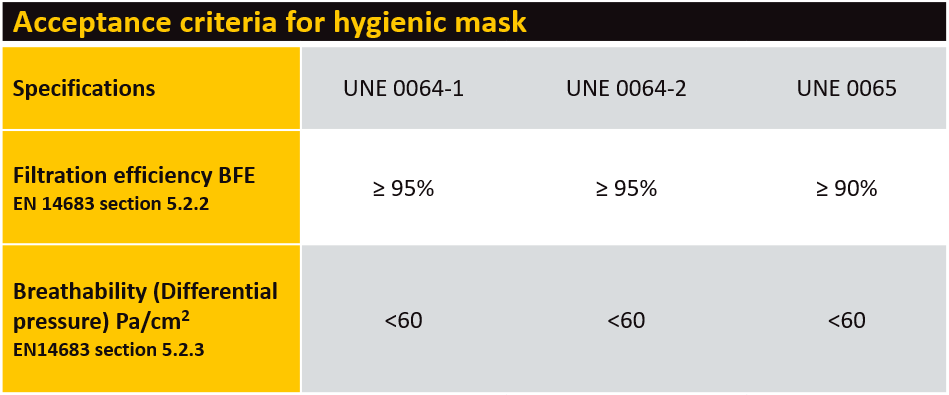
Tests for hygienic masks should be performed by ISO/ IEC 17025 accredited laboratories or accredited by an accreditation body that is a signatory to the mutual recognition agreement of the International Laboratory Accreditation Cooperation (ILAC).
Intertek PPE Resources
On-Demand Webinar: Introduction to Safety PPE Testing and Certification
Did you know the largest PPE market according to its end use is still PPE for the manufacturing industry, followed by construction? Download our webinar to learn more about Safety PPE Testing and Certification now.