Australian Industrial Chemicals Introduction Scheme (AICIS) in Effect
03 Aug 2020
Highlights of the AICIS, Annual Record-Keeping & Reporting Requirements and Transitional Rules
On 01 July 2020, the Australian Industrial Chemicals Introduction Scheme (AICIS) came into effect, replacing the National Industrial Chemicals Notification and Assessment Scheme (NICNAS). The New Australian Industrial Chemicals Law is now known as the Industrial Chemicals Act 2019. The AICIS is a regulatory scheme under the Industrial Chemicals Act 2019, formerly known as the New Australian Chemicals Law.
The aim of the AICIS is to make it easier for companies to import low-risk chemicals that are new to the Australian market.
Chemicals in Australia are regulated according to their use. The AICIS regulates chemicals with industrial uses. Chemicals for therapeutic, food, agricultural, or veterinary uses are not considered industrial chemicals, so they are therefore exempt from the AICIS. The aforementioned chemicals are subject to other regulatory requirements.
To introduce a new industrial chemical in Australia, companies are still required to register their business before import/manufacture, and the chemical being introduced must meet the requirements of a category of introduction based on the level of risk to human health and the environment. As well, a manufacturer or importer of an industrial chemical must register themselves with the Register of Industrial Chemical Introducers before introducing an industrial chemical during that year.
The AICIS sets out the categories of the introduction of industrial chemicals based on the level of risk to human health and the environment. It also sets out requirements that must be met for each category. Under the new scheme, the 6 categories of introduction are:
- Listed introductions
This category refers to chemicals that are listed on the Inventory and whose introduction is within the terms of the listing, if any. The chemical can be introduced without notification if the introducer is registered with the government. The obligations under the annual declaration and the obligations for record-keeping for listed introductions must be met. If a chemical is listed on the Inventory and has obligations/restrictions on the defined scope of assessment, conditions of introduction or use, or if the chemical has specific information requirement(s), the introducer must check if their introduction meets requirements; if met, the introduction is categorized as "listed."
- Exempted introductions (very low risk)
This category may include chemicals that are imported and subsequently exported, chemicals that are only used for research and development in certain volumes, polymers that are comparable to listed polymers, chemicals that are comparable to listed chemicals, and polymers of low concern.
- Reported introductions (low risk)
This category may include chemicals that are only used for research and development or low‑risk flavor or fragrance blend introductions.
- Assessed introductions (medium to high risk)
This category includes chemicals that are identified as having an indicative risk for either human health or environment or both of them that is medium to high.
Once the indicative health and environmental risk is determined if it is deemed that the category is medium to high risk introduction then an assessment certificate is needed prior to introduction.
- Commercial evaluation introductions
This category includes chemicals for which the applicant's intention is to evaluate the chemicals commercial potential and test the market viability of the chemical before full introduction. - Exceptional circumstances introductions
This category includes introductions requiring Ministerial authorization to allow for urgent introduction to protect public health or the environment.
Low risk introductions (i.e., exempted and reported introductions) can be made without assessment, while medium-to-high risk introductions must be authorized and require an assessment certificate issued by the regulatory authority.
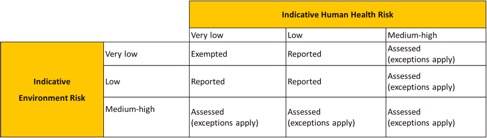
Manufacturers and importers must work out the indicative human health risk and environmental risk using the matrix bands below; this will provide the appropriate introduction category.
Reporting and Record-Keeping Requirements
Introductions made during a registration year and the category of those introductions must be declared to the authorities via an annual declaration. One declaration covers all chemical introductions in the year; separate declarations for each chemical introduction are not required. For chemicals that fit under the scope of reported introductions, a one-off pre-introduction report must be submitted to the regulatory authority before introducing an industrial chemical in that category.
The first annual declaration is due by 30 November 2021. It will cover the period from 01 July 2020 to 31 August 2021. After this, the registration year applies (September 1st to August 31st), and an importer/manufacturer will need to submit a declaration every year. The level of reporting is proportional to the risk level of the introduction, and the reporting obligations apply to all 6 introduction categories. Under the AICIS, an importer/manufacturer does not need to submit an annual report this year (i.e., 2020).
Transitional Rules
Transitional rules cover how certificates, permits, and processes under the old scheme will operate now. A commercial evaluation chemical (CEC) permit will transition into the category of 'commercial evaluation authorization' under the AICIS, and its terms and conditions will stay the same and will last as long as the NICNAS permit was in effect. All other NICNAS permits in place as of 30 June 2020 automatically became AICIS assessment certificates on 01 July 2020; these certificates have the same terms and conditions as under NICNAS. These certificates will be of a limited duration – either the time left on the original permit or until 30 June 2022, whichever date is later. There will be at least 2 years to re-categorize the introduction to ensure continued compliance with the new scheme once the transitioned certificate expires.
Introductions under NICNAS exemptions until 31 August 2022 may resume (i.e., R&D [less than 100 kg], no unreasonable risk in cosmetics [less than 100 kg], no unreasonable risk in non-cosmetics [less than 100 kg] and non-hazardous in cosmetics [less than 1%]). After 31 August 2022, categorization under the AICIS will be required.
Need Assistance?
Do you have questions about this topic, the changes being implemented to NICNAS/AICIS, or a related topic? Contact our experts at Intertek, we're here to help!

Kal Bening,
Regulatory Manager, Chemicals Group
Health, Environmental & Regulatory Services (HERS)
Today's expert blogger is Kal Bening. Kal is a Regulatory Manager and has been at Intertek for 16 years. Working with numerous clients, Kal's primary focus and role include providing clients with timely and cost-effective regulatory strategies under the various new and existing chemical notification programs. Her breadth of expertise centers around providing regulatory and scientific advice to clients to promote compliance with the New Substances Notification Regulations (NSNR) under the Canadian Environmental Protection Act (CEPA), the Australian National Industrial Chemicals Notification and Assessment Scheme (NICNAS), and other similar initiatives around the world in countries such as China and South Korea. Kal attended the University of Toronto where she received a B.S. in Integrative Biology & Environmental Sciences.