The Government of Canada’s Approach to Promote Transparency in Chemicals Management

07 Jun 2019
Can I Tell You a Secret?
There is so much happening in Canada on the topic of Transparency and release of Confidential Business Information (CBI) that this blog will need to be a little longer than usual to cover it all. This blog is intended to clarify the rights and obligations of industry stakeholders and the Government's responsibility and intentions moving forward.
As most of you know, in 2017 the Government of Canada, represented by Environment and Climate Change Canada and Health Canada, developed an approach to disclose CBI and promote transparency in Chemicals to achieve an appropriate balance between transparency and industry's right to protect its CBI.
What you might not have known is that the Government updated and finalized this approach in 2018 and updated their website (see the link below in the references section). The updated approach is meant to minimize the scope, frequency and duration of confidentiality claims for information related to substances listed on the Domestic Substances List (DSL).
How do I Claim Confidentiality?
First, let's discuss when a substance's identity or the information submitted to the Government should be claimed as confidential. You should only claim confidentiality when the information is truly confidential, such as when it's a trade secret or where its disclosure could negatively impact your competitive or financial position in Canada. If you are claiming confidentiality, you should provide a rationale for the confidentiality claim such as:
- It is a trade secret;
- It is information of a financial, commercial, scientific or technical nature that is treated consistently in a confidential manner;
- Its disclosure could reasonably be expected to result in material financial loss to the submitter or gain to competitors, or could reasonably be expected to prejudice the competitive position of the submitter; or
- Its disclosure could reasonably be expected to interfere with contractual or other negotiations of the submitter.
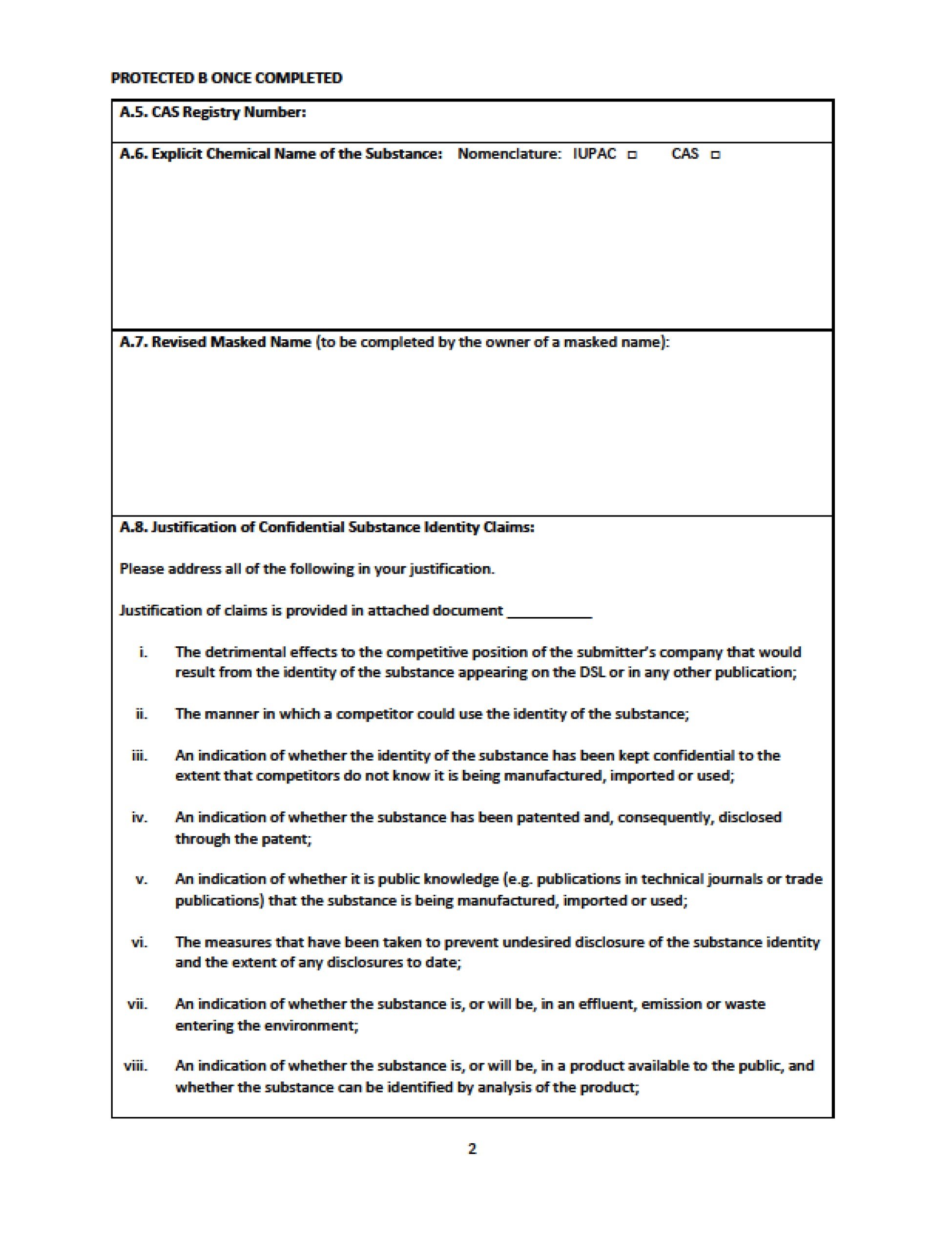
The claim for confidentiality, including a masked name proposal in the case of confidential substance identity claims, must be provided at the time of the notification in the case of new substances or nomination when proof exists that a substance was in the Canadian marketplace between 1984 and 1986 (existing substances) and the Government reviews the rationale. If the Government does not concur, they may reject the claim or contact the person submitting the information to request clarification or re-masking of the substance identity
Could There be Disclosure of Confidential Information?
Absolutely! There may be instances where the Government would wish to release certain CBI publicly. If so, the Government must be able to show that the de-classification and release of the information is in the interest of public health, public safety, or the protection of the environment or that it is necessary for the purposes of the administration or enforcement under the Canadian Environmental Protection Act 1999 (the Act).
In these situations, a reasonable attempt will be made by the Government to contact the person that was identified as the submitter of the CBI for any given company to discuss or negotiate what information, if any, will be released. The Government must show that the public interest in the disclosure clearly outweighs in importance
- Any material financial loss or prejudice to the competitive position of the person who provided the information or on whose behalf it was provided, and
- Any damage to the privacy, reputation or human dignity of any individual that may result from the disclosure.
This person that provided the information, or the person that has replaced this person will be asked to review and provide the appropriate rationale for their claim for confidentiality OR recommend appropriate masking (e.g., according to the Government's policy - see the Masked Name advisory note link below). As per the Act, at least 24 hours prior to disclosing any information, the Government will make a reasonable attempt to contact the submitter so that they are aware of the release of CBI.
What Information is Generally not Considered CBI by the Government?
The Government has identified certain types of information that they do not generally expect to be confidential, the release of which is seen as desirable to promote transparency. For example:
- Trade name(s) or name(s) commonly used (in cases where claims have been made to keep the substance identification confidential there will be no tie to these names);
- General information on uses;
- Safe handling precautions to be observed in the manufacture, storage, transport and use of the substance;
- Recommended methods for disposal and elimination;
- Safety measures in case of an accident;
- Physical and chemical information with the exception of data revealing the substance identity (e.g. spectra). If the physical and chemical information make it possible to deduce the substance identity, non-confidential ranges of values can be identified; and
- Summaries of health, safety, and environmental data including precise figures and interpretations.
Once again, if any of these have been treated as confidential by your company, a justification can be submitted to the Government when notifying or in response to a request asking to disclose.
Is There an Expiration Date on CBI Claims for Substance Identity?
Unfortunately, yes. Up until 2019, there was no limit for confidential claims for substance identity. However, in an effort to increase awareness of the substances in the Canadian market, the Government has implemented a 10-year review period on substance identity confidentiality claims.
Before the 10-year period has expired, the substance identity will be reviewed, and the Government will make reasonable attempts to contact the submitter at least 30 days prior to the expiry date to request that they update their claim for an additional 10-year period, if necessary. In case of the substance identity masking, the Government may ask that the masked name be updated according to the Masked Name Regulations (see the Masked Name Regulations link and advisory note link below).
With respect to the Government making reasonable attempts to contact the original submitter, this new confidentiality sunset policy further highlights the need to ensure that the "transfer of ownership" provisions are followed during company divestiture and acquisition activities. To ensure that the Government is able to reach the interested party, all notifications submitted by an acquired company should be transferred, whether they are interim schedules allowing required annual volumes or final schedules with full DSL listing. The successor corporation must provide the Government with a Certification Form (see the Interpretation of "Person" Advisory Note Link Below) in order to transfer certain rights in respect to substances, including ownership from the original notifier to the successor corporation and update the contact information of the person responsible for responding on any notices in respect to substances.
When is this Coming into Force?
The approach has taken effect in 2019 and the Government has started to contact the submitters of substance identity CBI claims of substances on the DSL that are older than 10 years. A flow chart was developed to explain the different steps that the Government was going to use (see the flow chart for handling older CBI claims link below).
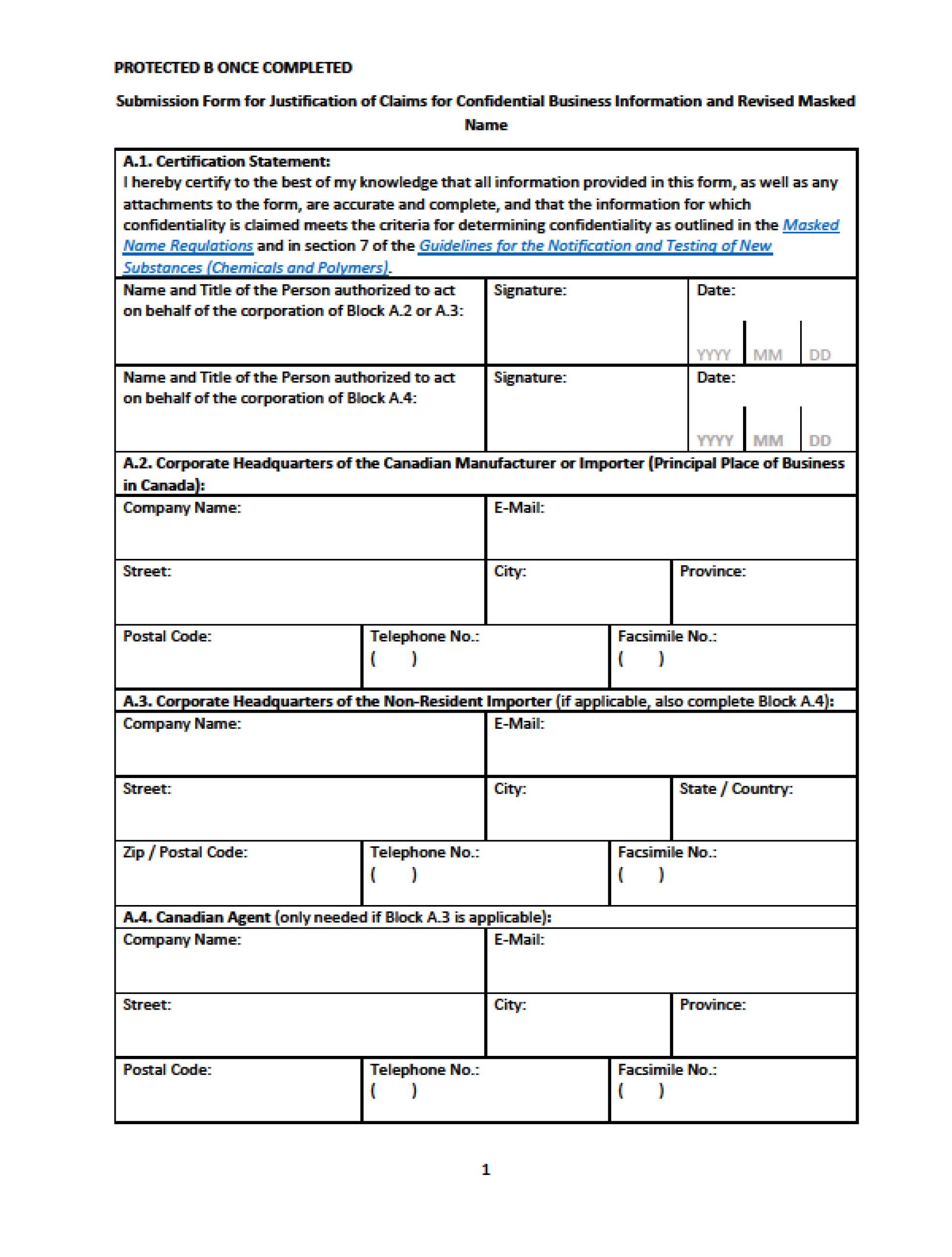
At this time, the Government is only focusing on the 23 substances in the pilot phase. If you, or the person that submitted the information, have received a letter from the Government and you wish to keep the substance identity confidential, you should use the response form that was created to facilitate these communications (see appendix 1 of this blog).
What Happens Now?
The Government explained that the de-classification of substances will occur in stages. The Government will:
|
Stage 1: |
Communicate with persons that have submitted confidentiality claims more than 10 years ago or that will soon reach the 10-year expiry date; |
|
Stage 2: |
Analyze the responses (or no responses) and prepare a Notice of Intent (NOI) that will be published; |
|
Stage 3: |
Publish a NOI with a 30-day public comment period with the names of the substances for which no response from the nominator was received or for which the Government rejected the justification of the nominator. The NOI will also request a CAS Registry Number for the substances that had none at the time of publication. Please note that the Government is looking at different options if a CAS Number is not provided. The Government will then re-evaluate the CBI justification taking into account any comments received in response to the NOI. |
|
Stage 4: |
Remove certain substances from the confidential portion of the DSL and add them to the public portion of the DSL in the Canada Gazette, Part II. For substances that will remain on the confidential portion of the DSL, the masked names will be updated in the Canada Gazette, Part II. |
What, if Anything, Should I Do Right Now?
Obviously, if you have received a letter from the Government, you should reply. The Government is requesting that replies be sent through its Single Window Information Manager Web application if you intend to submit justification to keep the CBI claim. Otherwise, email responses are accepted.
If you have not received a letter from the Government, please keep an eye out for the NOI that will be published, identify any substance you have an interest in keeping confidential and respond to the NOI within the 30-day comment period.
Conclusion
Do you have questions about this topic, the changes being made to the duration of substance identity CBI claims, the Chemicals Management Plan (CMP) or a related topic? Contact our experts at Intertek. We're here to help!
References:
The Canadian Environmental Protection Act, 1999
https://laws-lois.justice.gc.ca/eng/acts/C-15.31/index.html
2018 Approach to disclose confidential information and promote transparency in chemicals management
https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/approach-confidential-information-chemicals-management.html
Masked Name Regulations
https://laws-lois.justice.gc.ca/eng/regulations/sor-94-261/index.html
Interpretation of "Person" Advisory Note
https://www.canada.ca/en/environment-climate-change/services/managing-pollution/evaluating-new-substances/biotechnology-living-organisms/advisory-notes/march-1996.html
New substances: advisory note January 2015 - Masking substance names for New Chemicals and Polymers under the Canadian Environmental Protection Act, 1999, when the substance identity is claimed as confidential
https://www.canada.ca/en/environment-climate-change/services/managing-pollution/evaluating-new-substances/biotechnology-living-organisms/advisory-notes/january-2015.html
The flowchart to validate older CBI claims
https://www.canada.ca/content/dam/eccc/documents/pdf/pded/cbi-flowchart/Confidential-business-information.pdf
Environment and Climate Change Canada's Single Window Information Manager
https://ec.ss.ec.gc.ca/en/cs
How to report using the Single Window Information Manager: guidance
https://www.canada.ca/en/environment-climate-change/services/reporting-through-single-window/guidance.html
Appendix 1

Dan Bastien
Associate Director - Chemicals Group
Health, Environmental & Regulatory Services (HERS)
Today's expert blogger is Dan Bastien. Dan is the Associate Director of the Intertek Chemicals Group and is well known for his ability to effectively characterize and communicate the impacts of the regulatory environment on the chemical Industry. Dan is a subject matter expert in Canada with specific experience in the Chemical Management Plan (CMP), which includes Canada's New Substances Notification Program and the Assessment of Existing Substances. He has presented on these topics at numerous conferences around the world, held training sessions for the chemical industry, and co-authored guidance documents and other types of publications in Canada. Prior to joining Intertek, Dan managed, for over 20 years, the Client Services Unit of the New Substances and the evaluation of Existing Substance programs under the CMP. This makes Mr. Bastien uniquely qualified to provide practical, best-in-class service to help meet and understand Global Chemicals Management requirements.