To ensure the health and safety of users, personal protective equipment (PPE) for sale or use in Europe must comply with requirements set forth by Regulation (EU) 2016/425, as amended.
The aim of Regulation (EU) 2016/425 on personal protective equipment is to establish common standards for personal protective equipment (PPE) in all Member States in terms of protection of health and the safety of users, while enabling the free movement of PPE within the EU.
The regulation covers the design, manufacture and marketing of personal protective equipment and defines legal obligations to ensure that PPE on the EU internal market provides the required level of protection against risks.
In the EU regulation, PPE is defined as clothing or equipment worn or held to protect against injury or infection (including related interchangeable components and related connection systems ) with very few exceptions (i.e. PPE used by armed forces, certain private uses of PPE, and those subject to other rules like PPE on seagoing vessels, etc.)
Regulation 2016/425 replaced the previous PPE Directive 89/686/EEC from 21 April 2018. From 21 April 2019 the PPE Regulation is the sole legal instrument applicable for product s in its scope to be placed on the European Union market.
The regulation divides all PPE into three different categories according to the degree of risk (ANNEX I ), with Category I covering minor risks and Category III lethal hazards and irreversible damage. Depending on the risk category, different conformity assessment procedures must be followed to verify the conformity of the equipment.
For PPE equipment in Categories II and III, EU type examination (module B), which is part of the conformity assessment procedure, shall be carried out by a Notified Body.
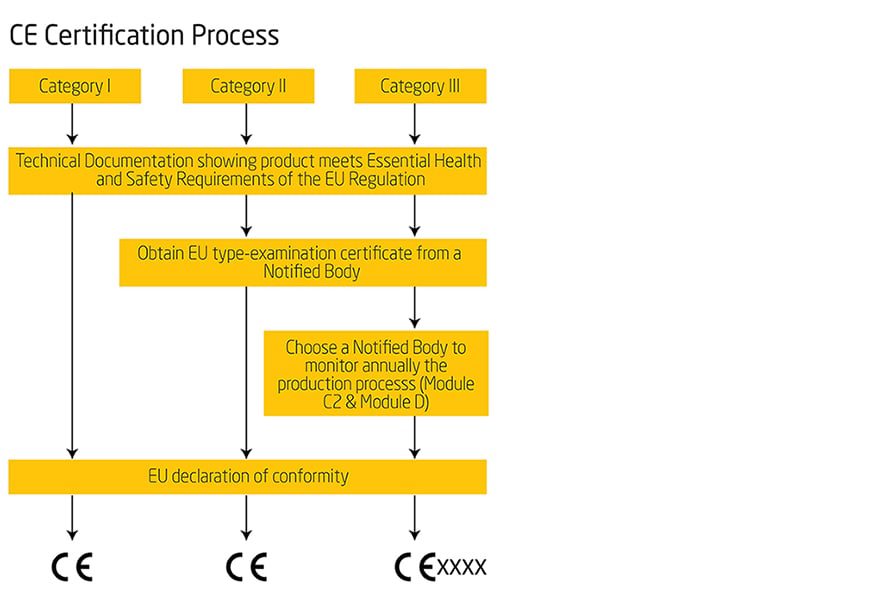
Where compliance of PPE with the applicable essential health and safety requirements (EHSR) has been demonstrated by the appropriate procedure, manufacturers shall draw up the EU declaration of conformity and affix the CE marking. They must keep the technical documentation and the EU declaration of conformity for 10 years after the PPE has been placed on the market.
The CE marking is mandatory and must be affixed before any PPE is placed on the market.
The regulation lays out the responsibilities and obligations of economic operators, including authorised representatives, importers and distributors, for ensuring that PPE products placed on the market are safe and compliant. It also specifies that technical documentation drawn up by manufacturers must be available for inspection by the competent national authorities for market surveillance.
Thanks to our strong technical expertise, with over 40 years of experience in PPE testing, inspection and certification, Intertek has 14 different PPE centres of excellence around the globe. As Notified Body for Regulation (EU) 2016/425 on personal protective equipment, we can help you bring your PPE equipment to the EU market.
Contact us today to verify the conformity of your PPE with the EU regulation.
From raw materials sourcing, to production, to retail, Intertek's end-to-end Total Quality Assurance solutions safeguard the quality and safety of a wide array of PPE and their supply chains with unmatched strengths. From raw materials sourcing, to production, to retail, Intertek's end-to-end Total Quality Assurance solutions safeguard the quality and safety of a wide array of PPE and their supply chains with unmatched strengths.


