Remote audits or virtual audits that help you to maintain control over quality and boosts visibility of your pharmaceutical drug supply chains. Delivered by our global network of expert auditors.
A remote audit or virtual audit is a viable means to maintaining control over quality across pharmaceutical or healthcare product supply chains when obstacles prevent or restrict a face-to-face, on-site audit. Recently, the pharmaceutical industry has embraced virtual audits to overcome the challenges related to travel restrictions due to COVID-19 safety issues, possible facilities closures to visitors and social distancing measures. Despite these restrictions, pharmaceutical supplier and subcontractor audits remain necessary to ensuring compliance across your supply chain.
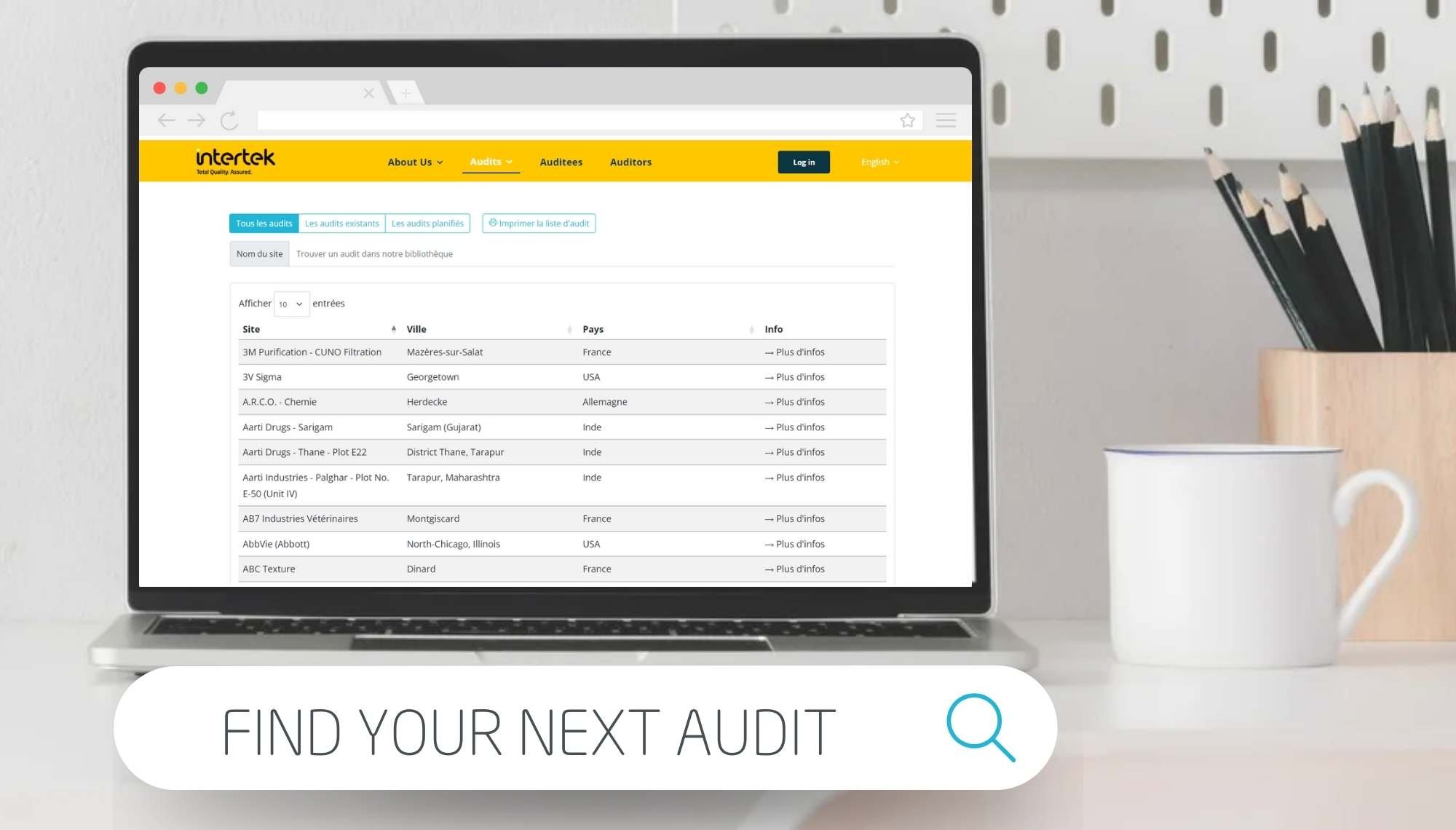
Remote Audit Solutions
Our virtual audits team aim to replicate a physical on-site audit including the same activities, as close as possible, as an on-site audit. With experience, our team can guide the auditee team on best practices to optimise each virtual audit covering open and closeout meetings, a facility tour, exchange of documents, document review, discussions with experts within the auditee team. We know that your focus is firmly on accelerating timelines for commercial release and so our auditors focus on the need to support and enable your audits program by deploying or conducting remote audits for your widespread supplier audits sites with consistent attention to detail and quality and professional stewardship.
Benefits of Remote Audits
Our remote audit solutions empower us to carry out activities efficiently and effectively, no matter the time or location. Whether conducted entirely remotely or in combination with on-site audits, these options offer significant time and cost savings, enhanced access to expertise, broader coverage, and reduced risk. Throughout the process, we maintain the same level of commitment and quality you rely on.
Auditing Expertise for Healthcare Supply Chains
Our audits activity was created over 15 years ago and has grown according to adapt to your audit requirements across the pharmaceutical, consumer healthcare, medical device and cosmetics sectors. Today we have a network of more than 130 qualified auditors, located across 5 continents and have gained the trust of over 700 client organisations. We conduct audits to address all types of suppliers and subcontractors for APIs, excipients, packaging articles, finished products, analysis laboratories, CROs, pharmacovigilance, transporters and distributor, cleaning subcontractors and IT service providers.
We conduct virtual audits against current relevant guidance documents for the pharma, medical device or consumer healthcare regulatory texts or standards such as Good Manufacturing Practice (GMP), Good Distribution Practice (GDP), Good Laboratory Practice (GLP), Good Clinical Practice, (GCP), Good Pharmacovigilance Practice (GVP). We also audit against IPEC guidelines for Pharmaceutical excipients, ISO 22716 (Cosmetics — Good Manufacturing Practices), EFfCI GMP for cosmetic ingredients, ISO 15378 standard for packaging materials, ISO 13485 standard for Medical devices, ISO 9001 standard or EHPM Quality guide for food supplements.
Total Quality Assurance
We are ISO 9001 certified. Quality is at the heart of our organisation and we continuously focus on improving the performance of our services in order exceed expectations of our global clients. With Intertek as your audit partner, we help you to overcome the challenges of auditing through a range of flexible auditing services. Alongside remote audits, we also provide in-person private or individual audits, shared audits, CAPA evaluation and follow-up programs, audit report purchase and support for your internal audit program. Bringing quality and safety to life, our auditors’ precision, pace and passion helps you to identify and mitigate the intrinsic risk in your operations, supply chains and processes helping you to power ahead.
Subscribe now
Subscribe to the Intertek Shared Audit newsletter