Bioanalysis and Immunogenicity Series - Volume 3
25 Sep 2018
Technologies for Measurement: A Case Study
Assessment of immunogenicity is a regulatory requirement and it's important to ensure the safety and efficacy of therapeutic protein products. Traditionally, immunogenicity assessment has been conducted via Enzyme Linked Immuno-Sorbent Assay (ELISA) and Electrochemiluminescence (ECL) technologies, which allow for precision, sensitivity, and drug tolerance. However, there are disadvantages to these techniques, such as a dependence on rigorous wash steps, which affects the ability to detect low affinity antibodies.
Given these disadvantages, alternative technologies and approaches should continue to be considered for immunogenicity assessment. Surface Plasmon Resonance (SPR) Bio-Sensors offers a label-free, real-time, direct detection system. SPR occurs when polarized light, shone through a prism, hits a metal film at the interface of media with different refractive indices. During the process, a molecular partner is immobilized on the metallic film and light excites surface plasmons in the metal. When the binding partner binds to the immobilized molecule there is a detectable change in the surface plasmon signal. To better see how SPR might serve as an immunogenicity assessment, we performed a case study.
To establish whether SPR was a suitable method to aid in immunogenicity assessment, we used infliximab, an approved biosimilar to treat rheumatoid arthritis. The study compared a bridging ECL format to an SPR format to detect anti-infliximab antibodies in rheumatoid arthritis human serum. Both methods were also compared to FDA draft guidance for immunogenicity assay validations, which establishes criteria for an assay to be deemed acceptable. In this case study we directly compared four validation parameters: cut-point, sensitivity, drug tolerance and precision. The results were:
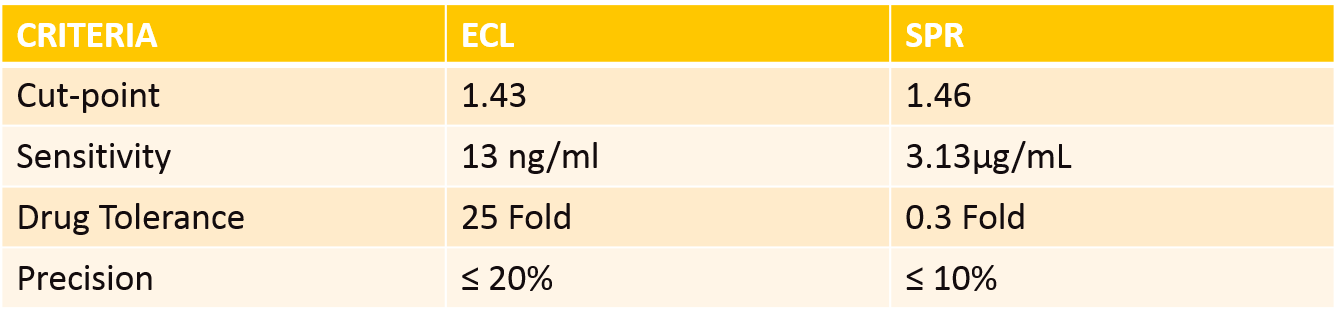
The ECL method overall performed better in the validation; however, there are other important factors to consider. One main advantage of SPR is its ability to detect low-affinity antibodies, something that traditional ELISA and ECL methods may struggle with due to wash steps. Although SPR techniques do not always meet the required sensitivity level, they can increase the number of positive samples detected. The SPR method is also a direct detection system, not relying on a bridging format or immunoglobulin detection reagents.
Some SPR instruments can perform automated acid dissociation steps, leading to observations of increased drug tolerance and a subsequent increase in positive samples detected. The main advantage of SPR-based detection over traditional techniques is the technology's ability to provide in-depth characterization of the ADA response. SPR technology is well suited to conduct isotyping, epitope specificity and relative dissociation rate (Kd) studies.
Immunogenicity is an important and complex factor to consider in the development of biological therapeutics for scientific, clinical and regulatory reasons. It is therefore important to consider assay platforms when designing studies, to ensure all positive samples are detected. The above case study highlighted that bridging formats with ECL endpoints are still the industry gold standard for ADA testing. However, it is important to consider a multitude of factors in the assay design. There are ways to overcome the documented drawbacks of less traditional technologies, and in fact, alternative technologies can offer some benefits, like the powerful characterization abilities of SPR.
For more insights on measuring immunogenicity and a closer look at this case study, download our complimentary webinar recording.
